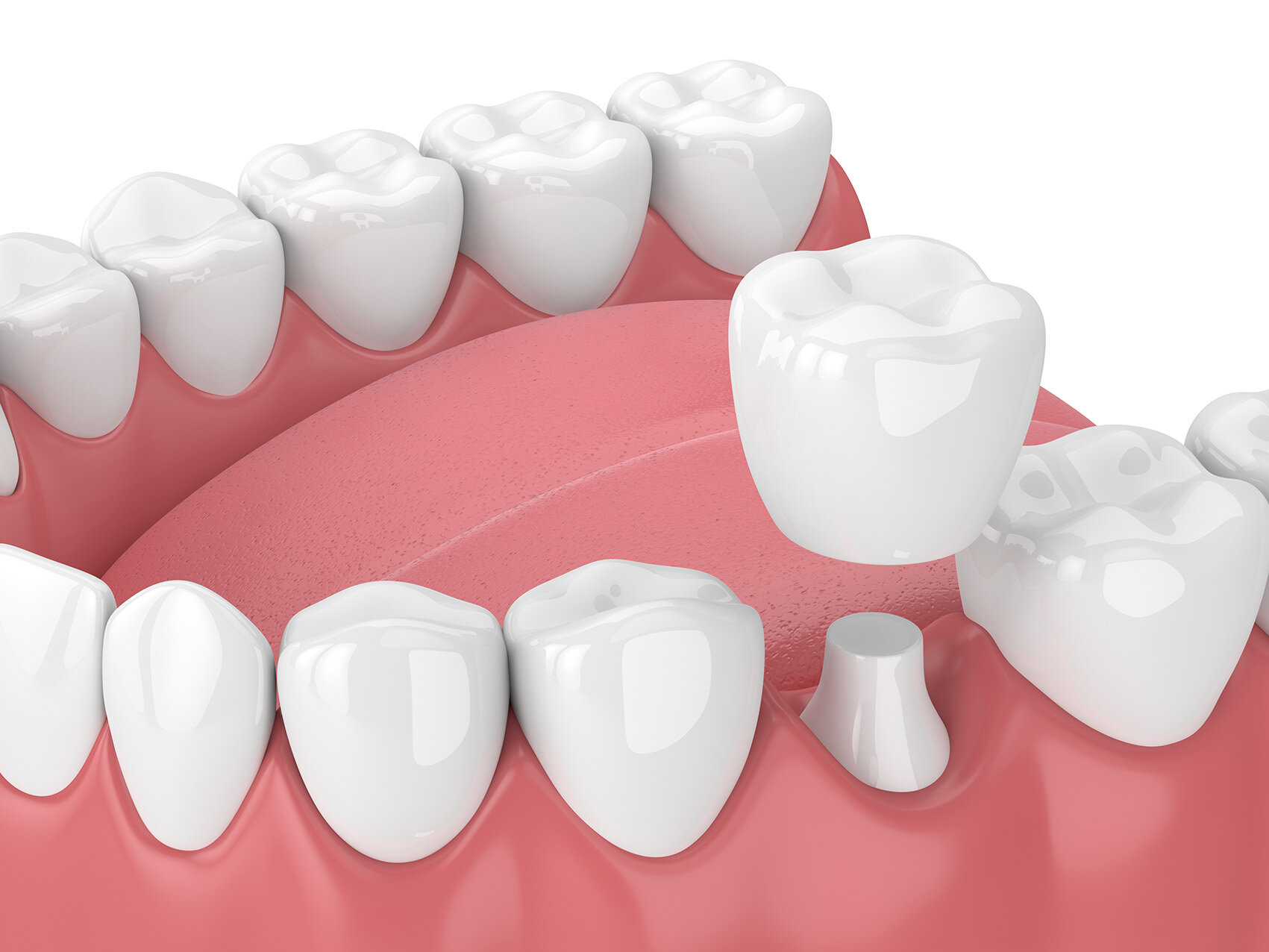
Crowns are custom-fitted caps that cover and restore damaged teeth. In addition, crowns help strengthen them to prevent further decay of the remaining enamel layers and further erosion of teeth.
Crowns are ideal for repairing chipped teeth and restoring those that have undergone root canal therapy, as well as supporting dental bridges or covering implants.
Porcelain
Porcelain is a ceramic material composed of two essential elements: kaolin (commonly referred to as china clay), which provides plasticity and structure; and petunse, an earthenware potter’s stone that gives porcelain hardness, translucency, and whiteness. Together these qualities give porcelain its special properties: low porosity density strength resistance thermal shock.
These properties make porcelain an excellent building material, as it is impermeable and easy to clean with high resistance against corrosion and chemicals. Furthermore, its insulating properties have made porcelain popular among electronic engineers while its strength makes it the material of choice for sinks, toilets and bathtubs – not forgetting dental crowns which must withstand pressure of bite forces.
Porcelain has long been seen as an emblem of refinement and class distinction, especially during history’s Ming Dynasty reign marked by an exceptionally smooth and glossy glaze that made Jingdezhen porcelain an imperial source of pride. Emperors would give porcelain urns to each other as gifts; best Jingdezhen porcelain became imperial pride. Yongle Emperor built a white porcelain brick-faced pagoda at Nanjing; its quality even distinguished him. The Ming Dynasty marked by this unique glossy glaze style which made an imperial prideful statement about class distinction and distinction that would endured into modernity.
In the seventeenth and eighteenth centuries, porcelain became an increasingly bourgeois material, finding its way into merchants’ hands and wealthy families’ households. Meissen and Vienna established prolific porcelain industries while England also prospered from this global industry. Bohemians became obsessed with this material known as “fine china,” making it an icon for middle classes alike.
Porcelain has long been used in tableware and decorative objects. Due to its hard, durable surface and natural colors that withstand harsh cleaning solutions, it makes an excellent material choice. Dental crowns and bridges often use porcelain. Unfortunately, however, porcelain may also crack or chip, at which time a dentist can restore the tooth using dental bonding or veneers that closely mimic natural teeth; sometimes cracked crowns require extraction and replacement with dental implants as more permanent solutions.
Zirconia
Zirconia is an extremely strong dental material with a natural white hue, which allows dentists to match it more easily for the ideal appearance. Thicker than metal materials like titanium and stainless steel, zirconia requires less healthy tooth structure to be removed during placement and stands up better against bruxism than crowns made with fired porcelain crowns.
Zirconium dioxide (zirconia) is similar to titanium in composition and even stronger. Like titanium, zirconia is completely biocompatible – meaning your body won’t reject it – making it an excellent option for patients with metal allergies. Zirconia is widely used for prosthetic hips, fingers and ears in medicine as well as dental implants, bridges, and crowns.
Zirconia crowns offer another advantage of zirconia: resistance to corrosion and staining. Traditional crowns may become discolored over time when exposed to food and liquid, which causes corrosion. Zirconia crowns don’t face this issue and will maintain their aesthetic appearance for many years to come.
One drawback of zirconia is its opaque quality. As such, crown makers sometimes combine it with porcelain when crafting crowns to increase aesthetics and color matching more easily; however, adding porcelain layers increases risk of chipping and delaminate, so this isn’t an optimal solution for everyone.
Zirconia and porcelain can also be combined in creating dental implants to make ceramic implants, which resemble natural tooth roots in appearance and can last decades. Furthermore, these dental implants help stimulate bone growth to ensure you maintain a strong jawbone.
Ceramic implants offer many advantages over metal ones, including being less costly. Ceramic implants make for an economical option when looking to recreate natural-looking teeth without breaking the bank for full dental restoration; additionally, ceramic is often favored by those missing teeth who wish to avoid dental bridges or dentures as a viable replacement option.
Base Metals
Base metals such as copper, zinc, nickel and tin are an invaluable part of everyday life, found everywhere from construction projects and transportation vehicles to industry applications and more. Their abundance means less expensive mining costs compared to more precious precious metals; hence their status as commodities.
Base metals, or nonferrous metals, contain no iron and tend to be easier and cheaper to mine than precious metals such as gold and platinum. While base metals may seem mundane for investing purposes, their many desirable qualities make them highly sought after among investors for various reasons.
Investors can trade metals in several ways. Some traders purchase physical commodities directly while others trade futures contracts – an agreement which specifies the price of a specific metal at some future date, giving investors an opportunity to speculate on changes in the market.
Given their many applications, base metals are frequently seen as indicators for overall economic health. Copper’s price can serve as an indication of residential homebuilding activity; when its prices increase, economists often interpret this as evidence that the economy is growing stronger while its decline indicates activity has decreased – both of these changes signal to economists that activity in critical sectors such as homebuilding may have decreased or may indicate declines.
Base metals have numerous commercial applications; they’re also often utilized in dentistry. For instance, certain crowns made of a mix of precious and base metals like porcelain fused to a metal core offer both strength and natural look for natural looking restoration. Most often seen on molars but can be used anywhere within the mouth.
Metal crowns can withstand biting and chewing forces well, and are less prone to chipping or breaking than porcelain ones. Unfortunately, however, they tend to be more costly than other dental crowns, and may give the appearance of having metallic coloration, which may not be ideal in front teeth.
Alloys
“Alloy” refers to any mixture of metals. Alloys are generally created by melting one metal and mixing it with others (sometimes including non-metals ) in order to improve its properties, and are found almost everywhere from doorknobs and utensils to medical instruments and even aircraft engines! Copper, brass, duralumin and nitinol are just a few common examples that we encounter everyday; others include aircraft engine components as well as tools or decorative articles used during building and home construction projects!
Alloys are immensely valuable because they provide properties not found in pure metals. These may include increased tensile strength, corrosion resistance or hardness that make alloys invaluable. Their properties make them well suited to many applications while simultaneously cutting costs by taking advantage of less expensive raw materials that would otherwise go to waste.
One of the primary uses for alloys is corrosion resistance. Their chemical composition prevents ions from penetrating their structures, thus protecting against contact between corrosion-forming elements and metal surfaces. Bronze is one alloy which stands out as particularly effective against corrosion; being made up of copper and tin it is much less likely to corrode than its counterpart, pure copper.
Another advantage of alloys is their adaptability; they can be tailored specifically to individual needs. For example, some alloys are used as resistance elements for controlling and measuring electrical current through wire-wound resistors, rheostats, potentiometers or potentiometry while thermocouples use different alloys to detect and record temperatures.
Alloys can also be found in medical and dental equipment, jewelry, coins, medals and other art objects, industrial and automotive applications as well as to improve metal properties – for instance titanium is often alloyed with aluminum or chromium in order to enhance corrosion-resistance and strength.
Disclaimer: The content on this blog is intended for general informational purposes only. It is not a substitute for professional medical advice, diagnosis, or treatment. Always consult qualified healthcare providers for personalized advice. Information regarding plastic surgery, dental treatment, hair transplant, and other medical procedures is educational and not a guarantee of results. We do not assume liability for actions taken based on blog content. Medical knowledge evolves; verify information and consult professionals. External links do not imply endorsement. By using this blog, you agree to these terms.